- TOP
- Corporate Information
- Project Story
- Bronchial Filler EWS Born from the Passion of a Doctor
A Phone Call
One day in 1999, the Medical Team (referred to below as the MED Team) received a phone call from a medical device distributor in Okayama. The call was about a request from a doctor who would like to have some help in commercializing a medical device. With the details of that request, we at the team approached a French company that we had been doing business with for some time, and they agreed to the commercialization. That should have been the end of it.
However, because it was a medical device with a new concept, medical approval could not be obtained without clinical trials to validate its efficacy and safety. On the other hand, if such clinical trials were conducted, it would cost around amount to 500 to 600 million yen. Furthermore, the market size was unknown at that point in time. Therefore, it was not a situation that we could easily get involved in. The MED Team was reluctantly involved by helping doctors to import the device individually with their own medical license without taking our profits. This carried on for over 10 years.
Bronchial Filler EWS Is Born
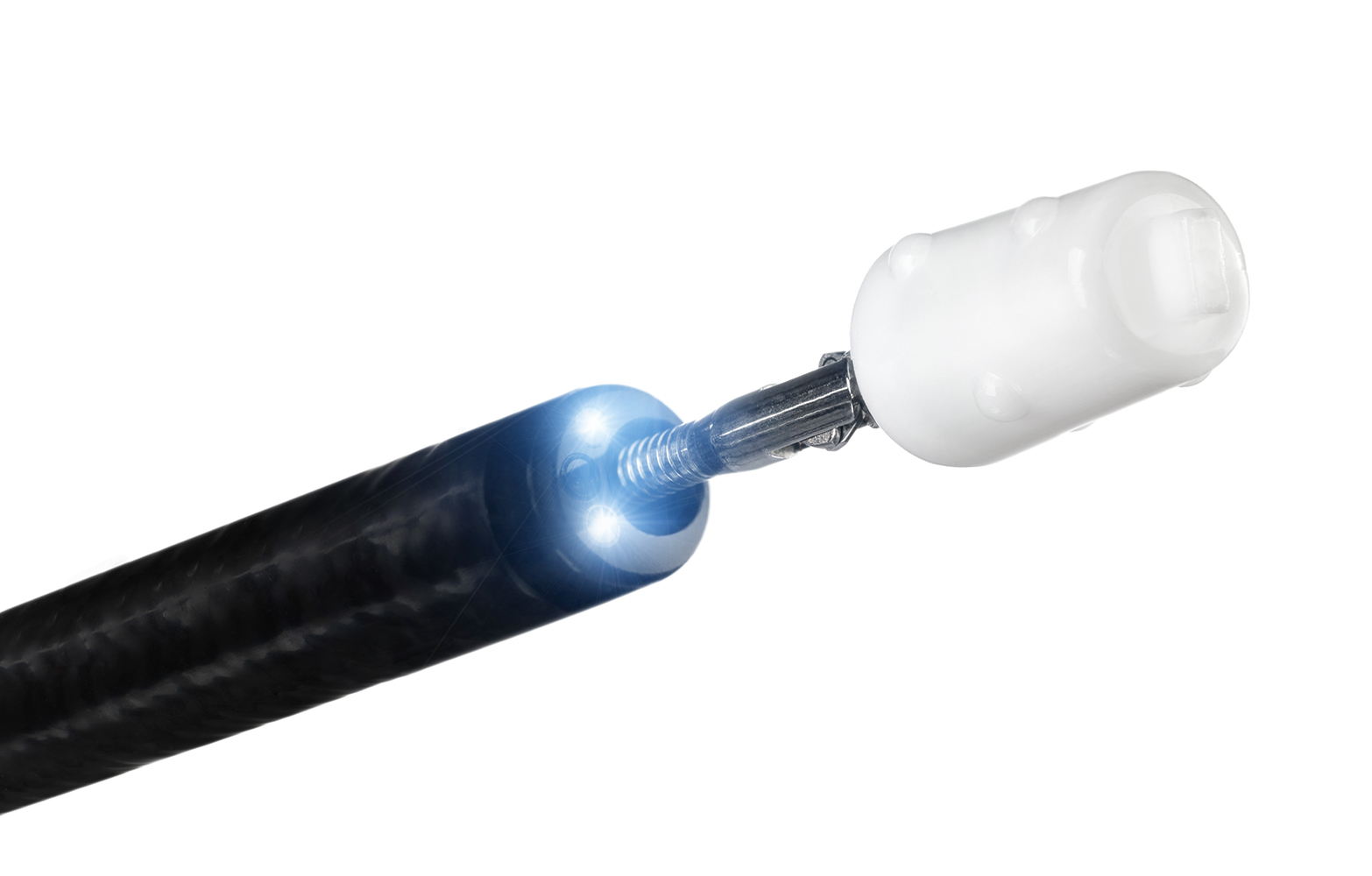
Dr. Watanabe was working at the Okayama Red Cross Hospital in 1989. At that time, he was treating a patient with sepsis. The patient needed respiratory support with a ventilator, but one day he had an opening in his bronchial tube, which caused air leakage into the kidney. In spite of various treatments for him, there would be no other situation than the patient was expected to have a poor diagnosis. Dr. Watanabe, after thinking it through, tried inserting a small plug handmade of dental silicone into the patient’s bronchial tube, and his condition improved steadily. Then, he was discharged from the hospital without any incident.
This was the beginning of the bronchial filler EWS (referred to below as EWS).

Subsequently, Dr. Watanabe treated patients with intractable pneumothorax with his handmade filler, which saved the lives of many of patients. Therefore, he came to consider commercialization of this filler to save more patients. In 1999, Dr. Watanabe approached a few Japanese companies manufacturing medical devices with silicone materials to commercialize his filler. However, they all turned down his proposal due to the aforementioned issues with clinical trials. Therefore, Dr. Watanabe, who thought that he should bring it to overseas companies if he had no chance with a Japanese company, approached Harada Corporation through a local distributor. Though it was uncertain whether the business would be successful or not, we believed that his plug would be able to save the lives of many patients. Then, when we consulted a French manufacturer that we had been dealing with for many years, they agreed to commercialize it. Immediately, we set up the visit to Japan of a person in charge and a meeting with Dr. Watanabe. After several trial products, the EWS was completed.
The number of EWS cases gradually began to increase through individual import by doctors from around 2000. In addition, as the effectiveness and safety of the EWS were presented at various medical conferences, cases of individual import with doctors’ licenses spread to hospitals nationwide. The EWS came to be recognized by many doctors over a period of four years.
However, there was a major obstacle to overcome for saving more patients: approval for medical devices. The EWS was not covered by insurance because it was not approved as a medical device. In other words, the more it was used, the more it would leave hospitals in the red. Dr. Watanabe hoped that the EWS would be covered by insurance to save the lives of more patients. Harada Corporation had the same spirit to some extent but could not give the go-ahead to requesting medical approval, considering the amount of money to be invested and the market size of the product. Nevertheless, we continued our persistent search for what the MED Team could do at the time.
Challenging the Biggest Obstacle
In 2008, we began to receive opinions from not only Dr. Watanabe but many other doctors that they wanted us to make the EWS available through insurance. Around that time, an MHLW official advised us that if the EWS was designated as a medical device for rare diseases, the company could obtain a subsidy of tens of millions of yen from the government and reduce the number of cases in the clinical trial. On the condition of being designated as said medical device for rare diseases, we, Harada Corporation, decided to start full scale procedures for the application of medical device approval.
Right away, we prepared the application, asking a consulting firm to collect the data. According to the consulting firm, there was a fifty-fifty chance that our application would be approved. The firm also held the view that the approval would not be easy since the applicant company was not a medical device manufacturer. As predicted, the process took a long time. However, our product was finally designated as a medical device for rare diseases in 2009. One year had passed since the application.
In 2010, the clinical trial was started with a subsidy from the government (the actual payment for expenses was made after the clinical trial was completed) in addition to the investment of Harada Corporation. The clinical trial was completed in 2011. Then, in 2012, we applied for medical device approval. We finally received this in 2013. Five years had passed since the project was given the green light in 2008.
However, it was not finished yet. The product came to be covered by insurance as it was approved as medical device. Meanwhile, Japan had a rule that the national health insurance reimbursement price for foreign-made medical devices could be accepted only up to one and a half times as much as the actual market price in foreign countries (i.e., the purchase price by hospitals). On the other hand, to recover its investment in the clinical trial, Harada Corporation had no choice but to set the selling price at a higher level than the reimbursement price. Even so, it was estimated it would take nearly 70 years for the company to recover its investment. In other words, the situation remained the same; the more it was used, the more it would leave hospitals in the red. Nevertheless, major hospitals nationwide used the EWS. This was a big challenge and a big decision for doctors who would use the product as well as the hospitals that allowed doctors to use it. The MED Team, believing that the doctors’ passionate wishes would come true, took advantage of presentations at academic conferences to continue to question the contradiction in the 1.5 times rule and to advocate for an increase in the reimbursement price.
In the meantime, something good happened to us. In 2016, an employee among the MED Team received the prestigious Miyazawa Prize from the Japan Society for Respiratory Endoscopy for his contribution to the development of respiratory intervention through the promotion of the Dumon stent, rigid bronchoscopy, and the EWS. It was the moment when we realized we were supporting doctors’ passionate resolutions, even if it was only slightly.
In 2018, our request on re-pricing unprofitable products, which had been submitted to the Ministry of Health, Labour and Welfare (MHLW), was approved even though we thought it would be impossible, and the reimbursement price for the EWS was raised to approximately three times higher than the then existing price, which made it easier for hospitals to use the EWS. Accordingly, the payback period for the investment by Harada Corporation was much shortened, too. Because it is customary to lower reimbursement prices to once every two years by a review of medical fee points, it was highly unusual to raise reimbursement prices by three times. We had made objection against the MHLW’s reimbursement pricing rules, and this was the moment when our extraordinary efforts bore fruit.

As a Partner Who Takes on Challenges Together
It took 24 years from the invention of the EWS, a bronchial filler by Dr. Watanabe, until it became applicable in Japan’s national health insurance system, and 29 years until our request for repricing unprofitable products was approved, so that finally the EWS could be supplied in the clinical environment at an appropriate price.
Dr. Watanabe recalled in his book that the path to insurance coverage was so long that his heart almost broke.* Receiving the news that the EWS was approved as a medical device, he stated that he was overjoyed from the bottom of his heart.
Dr. Watanabe passed away in November 2013 after he made sure that the EWS had obtained medical approval.
Currently, it may be said that the EWS has created a win-win situation for everyone.
・This is a rewarding treatment for doctors to be able to save patients.
・Patients’ quality of life will improve due to earlier discharge from hospital.
・Speeding up patient’s early discharge will enable the government to reduce medical costs.
・Harada Corporation can contribute to the community and will be able to recover its investment in the future.
In addition, this project broke the ice for Harada Corporation to become recognized by medical-related academic societies and doctors, which allowed us to develop new products easily.
Harada Corporation is neither a medical institution nor a medical device manufacturer. For that very reason, the mission of the MED Team is to save the lives of patients together with medical professionals on the frontline by taking in a lot of knowledge and information always with unconventional thinking, as well as through challenges that only a trading company can take on. The MED Team will continue to be a partner who we strive and grow with together to support the future of medical treatment.
* Source: Yoichi Watanabe, “Atarashii Iryo eno Chosen (in Japanese, meaning Challenges to New Medical Treatment),” pp. 44-52
PROJECT STORY
-

Rain Poncho for Leisure Facilities Produced as a Non-disposable Environmentally Friendly Product
“I want to enjoy water attractions, but don’t want to get soaked.” The rain poncho for leisure facilities was produced to meet the needs of such consumers. While most of those facilities use simple, disposable ponchos, these rain ponchos are becoming popular as they are high value-added and environmentally friendly products.




